Background:
Though the available number and efficacy of novel agents for multiple myeloma (MM) have improved in recent years, it remains an incurable disease with relapse inevitable in nearly all patients. Autologous stem cell transplantation (ASCT) as consolidation after induction remains the standard of care for transplant-eligible patients with randomized studies showing progression-free survival (PFS) greater than 4 years (Atttal et al., N Engl J Med 2017; 376:1311-1320).
Relapse outcomes are also improving with PFS now greater than 3 years in some settings (Bahlis, et al., Leukemia 2020 Jul;34(7):1875-1884). For fit patients with good response to a first ASCT, a second autologous transplant (SAT) can be considered. The American Society for Blood and Marrow Transplantation (ASBMT) and the International Myeloma Working Group (IMWG) guidelines suggest SAT should be considered for those whose first ASCT resulted in remission duration >18 months (Giralt et al., Biol Blood Marrow Transplant 2015; 21:2039-2051). CIBMTR data note 600-700 SATs are performed annually. Most data on SAT come from single-center retrospective series, though pooled analyses note median PFS >1 year and median OS >30 months (Hagen and Stiff, Biol Blood Marrow Transplant 2019 Mar;25(3):e98-e107).
Here we present our institution's experience with salvage SAT for MM analyzing treatment outcomes and toxicities.
Methods:
We performed a single-center retrospective review of all MM patients at Oregon Health and Science University who received SAT for myeloma as salvage therapy. Planned tandem transplants were excluded. Demographic and disease characteristic data at time of original diagnosis and at time of SAT were extracted and responses assessed by IMWG criteria. OS and PFS from SAT were calculated by Kaplan Meier method with progression or death from any cause counted as events for PFS and censoring at last known follow-up for live patients.
Results:
Baseline characteristics: Sixty-eight patients were identified who received SAT between 1999 and 2020. Durie-Salmon staging at diagnosis was available for 30 patients; 16 (24%) 3A, 7 (10%) 2A, 1 1B (1%), 6 (9%) 3b. The median age at time of SAT was 61 (range 45 -74). The median time between first and second ASCT was 5.5 years, (range 1.1 -15.2 years). Median PFS after first ASCT could be calculated for 53 patients and found to be 2.5 years (range 0.3 - 10). First ASCT conditioning regimen and dosing were available for 56 patients; 55 (81%) received melphalan with 53 (78%) receiving 200mg/m2, 2 (3%) receiving 140mg/m2, 1 (2%) received busulfan + TBI. The average number of lines of therapy prior to SAT was 2.8 (range 1-14). SAT preparative regimens were available for 67 patients: 59 (87%) received melphalan 200mg/m2, 6 (9%) received melphalan 140mg/m2, 1 (2%) received BEAM, 1 (2%) received melphalan 200mg/m2 and bortezomib. All SAT patients received peripheral blood stem cell mobilization.
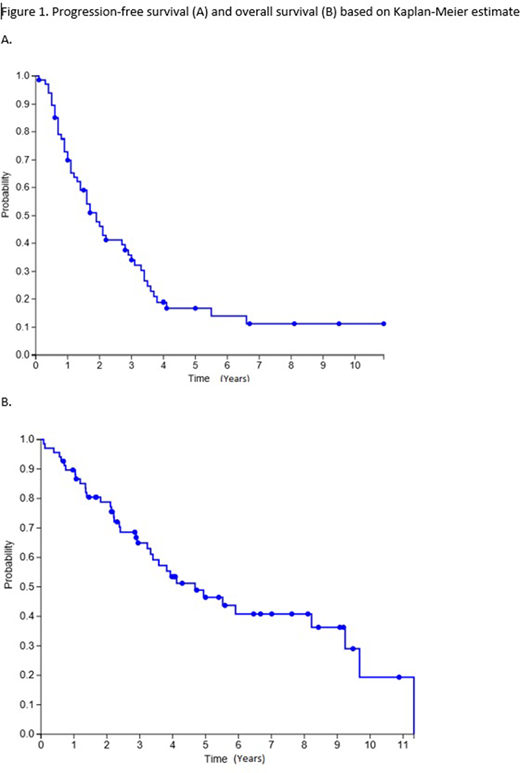
Outcomes: 37 (54%) patients have died at time of data collection. Median OS after SAT was 3.09 years while median PFS was 1.65 years. Treatment related mortality (TRM, defined as death not due to progression within 100 days of SAT) occurred in 1 patient (2%). Long term complications included 10 patients found with other malignancies after SAT (5 with basal or squamous cell skin carcinoma, 2 with MDS, 2 GI malignancies, 1 prostate).
Conclusion: Our institution's experience with SAT for MM suggests it's an effective salvage treatment with low treatment-related mortality, though with a shorter PFS than after first transplant. Most patients were able to receive and tolerate full dose melphalan 200mg/m2 for SAT.
Silbermann:Sanofi-Aventis: Consultancy, Research Funding; Janssen: Consultancy; Karyopharm: Consultancy. Maziarz:Incyte, Kite, BMS/Celgene, PACT Pharma, Orca BioSystems, and Omeros: Honoraria; Novartis and Juno: Research Funding; Novartis and Athersys: Other: DSMB participant; Novartis, Incyte, CRISPR Therapeutics, Artiva Biotherapeutics, and AlloVir: Consultancy; Athersys: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal